A convenient amount unit for expressing very large numbers of atoms or molecules is the mole. ___ refers to the sum of the atomic masses of all the atoms in the formula unit of an ionic compound.
Atomic Number Mass Number Definition Facts Videos Calculations With Examples And Faqs
Da where 1 amu or 1 u or 1 Da is defined as 112 of the mass of a single carbon-12 atom at rest.
. 11 Which term represents the sum of the atomic masses of the atoms in a molecule. Chemists deal with the masses of _____ of substances. The atomic mass is the weighted average of the masses of all isotopes of an element.
And electrons due to the loss of some energy due to binding energy mass loss. The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units. 19 3 OVER 12 Which equation represents energy being.
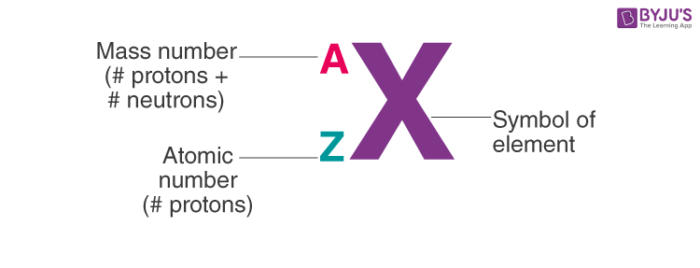
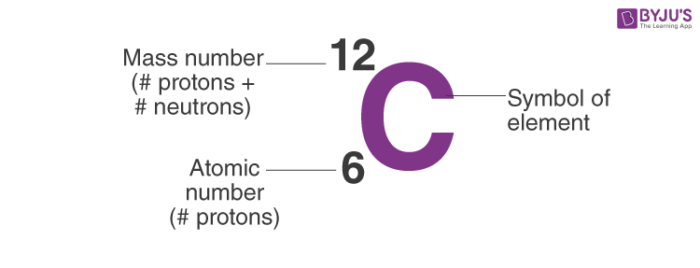
New book 21 Terms. The mass number of an atom is the sum of number of protons and neutrons it contains. 1 atomic number 2 mass number 3 formula mass 4 percent composition by mass.
The molecular mass is the sum of the masses of the atoms in a molecule. One atomic mass unit is equal to 166 x 10-24 grams. Hence to a first approximation the mass of an atom is the sum of the number of protons the atomic number Z and the number of neutrons Nie the mass number A Z N.
Although the chemical behaviour of each atom is uniquely identified by its atomic number Z the neutron number N and hence the mass number can vary within limits. Atomic Theory Quiz Study. Which term represents the sum of the atomic masses of the atoms in a molecule.
The sum of the atomic masses of all atoms in the formula unit of an ionic compound. The atomic mass ma or m is the mass of an atom. The formula mass of a substance is the sum of the average atomic masses of each atom represented in the chemical formula and is expressed in atomic mass units.
Atomic mass represents the mass of an atom which can only be one isotope. The molecular mass of SO 3 with 4 atoms in its molecule is 1S 1 x 3207 amu 3207amu. Atomic mass is the total mass of particles of matter in an atom ie the masses of protons neutrons and electrons in an atom added.
The mass number tells us the number the sum of nucleons ie number of protons and neutrons in the nucleus of an atom. Which equation represents energy being absorbed as a bond is broken. The unit of measure for mass is the atomic mass unit amu.
The mass number reports the mass of the atoms nucleus in atomic mass units amu. Because there is usually one isotope that is much more common on Earth rounding the atomic mass to the nearest whole number gives the atomic mass of that isotope and is called the atomic mass number. The sum of the atomic masses of all the atoms present in a molecule gives the.
The relation between the two units is. The sum of the atomic masses of all the atoms in a molecule. Log in Sign up.
The atomic mass unit u is a unit that describes the masses of individual atoms and molecules. The sum of the atomic masses of all atoms in the formula unit of an ionic compound is called the formula mass of the substance. Molecular mass is incorrect when applied to an ___ _______.
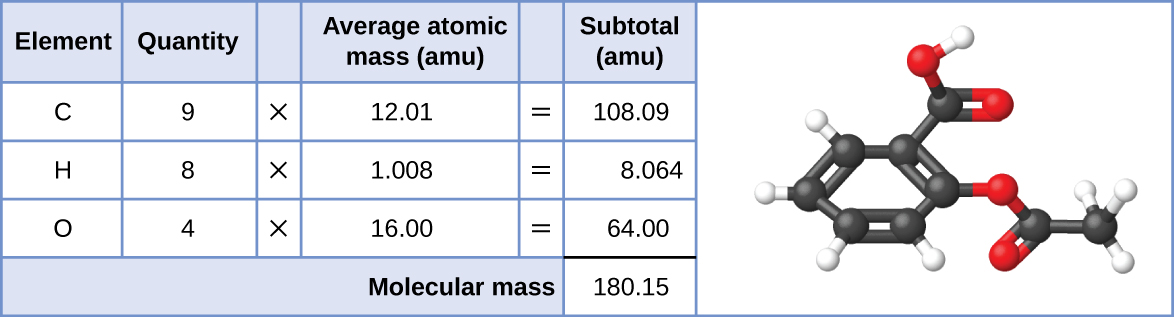
Besides the standard kilogram it is a second mass standard. Figure 63 The average mass of an aspirin molecule is 18015 amu. The formula mass of a compound is the 1 sum of the atomic masses of its atoms 2 sum of the atomic numbers of its atoms 3 product of the atomic masses of its atoms 4 product of the atomic numbers of its atoms.
Likewise the molecular mass of an aspirin molecule C 9 H 8 O 4 is the sum of the atomic masses of nine carbon atoms eight hydrogen atoms and four oxygen atoms which amounts to 18015 amu. The atomic number is the number of protons found in the nucleus of an atom. Which term is used to describe the attraction that an oxygen atom has for the electrons in a chemical bond.
One ___ represents the SI standard base unit of chemical quantity. One atomic mass. It is the carbon-12 atom which by international agreement has been assigned a mass of 12 atomic mass units u.
Learn vocabulary terms and more with flashcards games and other study tools. The formula mass of a covalent compound is also called the molecular mass. The formula mass of a covalent compound is also called the molecular mass.
Atomic mass represents the quantity of matter that is contained in the atoms of an element. - most of atoms mass and positive charge make up nucleus. The protons and neutrons of the nucleusaccount for nearly all of the total mass.
The molecular mass or formula mass of a compound is the sum of the atomic masses of all the atoms in the molecule. H2 energy H H. A convenient amount unit for expressing very large numbers of atoms or molecules is the mole.
Kg atomic mass is often expressed in the non-SI unit atomic mass unit amu or unified mass u or dalton symbol. As compared to the sum of the masses the atomic mass is slightly less. The average atomic mass of the atoms for fluorine are 18998.
The SI unit of atomic mass is the kilogram kg but it is often expressed in terms of non-SI unit. Start studying Unit 3. The method for finding the atomic mass is dependent on the purpose that either one has to look at the natural sample single atom or the atom containing some ratio of isotopes.
Reason The mass number of an atom can never be smaller than the atomic number. For example on the periodic table sodium Na has an atomic number of 11 and an atomic mass of 22990. The sum of the mass number and the atomic number for an atom A-Z corresponds to the total number of subatomic particles present in the atom.
Although the SI unit of mass is the kilogram symbol. It is traditionally represented by the symbol Z. It is expressed as 602 X 1023 units.

Atomic Number Atomic Mass And Isotopes Article Khan Academy
2 3 Calculating Atomic Masses Chemistry Libretexts
Atomic Number Mass Number Definition Facts Videos Calculations With Examples And Faqs
0 Comments